Standard Operating Procedure (SOP) for Self Inspection (Internal Audit) of various departments/sections and functions of the pharmaceutical drug manufacturing plant.
Self Inspection (Internal Audit) Procedure
1.0 Objective :
-
- To lay down the procedure for conducting the self-inspection (Internal Audit) in order to implementation of Quality Management System and compliance with GMP practice principles, Data integrity, and to propose necessary corrective and preventive actions.
2.0 Scope :
-
- This SOP is applicable to the Company’s operations directly or indirectly connected with
-
-
- Manufacturing,
- Stores,
- Dispensing,
- Sampling,
- Packaging,
- Quality Control,
- Microbiology,
- Engineering,
- IT,
- Personnel,
- Safety,
- Documentation, etc.
-
3.0 Responsibility & Accountability:
-
- All Department Heads: To follow the procedure
-
- Head – QA: For system compliance
4.0 Procedure for conducting Self Inspection (Internal Audit) :
-
-
Composition of self-Inspection team
- Head QA or his/her designee shall constitute the internal audit team from a cross-functional area, one member shall be from the QA department and other auditors shall be from any of the other departments.
-
-
- Selection criteria for auditors as follows:
-
-
- The auditor shall have the relevant educational qualifications and professional experience (Not less than 6 years) and able to perform the assigned tasks.
-
-
-
- Internal Auditor shall have area knowledge, Knowledge of method of inspection & assessment, and subject knowledge.
-
-
-
- The auditor shall have exposure to regulatory audits and knowledge of regulatory requirements.
-
-
- Head QA / Designee shall prepare the list of auditors in Annexure III, after discussion with Head Quality, before each inspection.
-
- The below-given training shall be provided to the auditors:
-
-
- Training on SOP of self-inspection.
-
-
-
- Training on inspection skills.
-
-
- Inspection team members shall be independent of those having direct responsibility for the area being audited.
-
-
Areas for inspection (but not limited to):
-
-
- Electronic record copy
-
- Human Resources.
-
- Storage of Raw materials, Packing materials, and finished products.
-
- Production and in-process controls.
-
- Quality control.

- Quality control.
-
- Quality Assurance.
-
- Labels control.
-
- Documentation.
-
- Sanitation and hygiene.
-
- Validation and revalidation programs.
-
- Calibration of instruments or measurement systems. (Related: SOP for Internal & External Calibration)
-
- Complaints management.
-
- Recall procedure.
-
- Premises including personnel facilities.
-
- Maintenance of buildings and machinery. (Related: SOP for Building Maintenance)
-
- Equipment.
-
- Results of previous self-inspections and any corrective steps are taken.
-
- Compliance of audits conducted by any External Agency and/or any regulatory body after the last internal audit.
-
-
Conduction of the self-Inspection
-
-
- The Head QA is responsible for all self-Inspection program arrangements, preparations, conducting, reporting and compliance towards corrective action.
-
- The Head QA shall discuss with the Inspection team in meeting and shall inform all departments through circular prior to conduct Inspection.
-
- The inspection shall be conducted as per a predetermined Inspection schedule as per Annexure-II.
-
- This Self-Inspection schedule shall be prepared in the Ist week of January for each calendar year.
-
- The inspection schedule shall be prepared by Officer/Executive QA, approved by Head QA.
-
-
Frequency of Internal Audit:
-
-
- At least two times a year, in the case of product recalls or repeated product/batch failure.
-
- The Self- Inspection checklist shall be updated so as to be in line with cGMP requirements and current laid down SOPs related to the activities.
-
- The Self-Inspection team shall audit individual departments like Stores, Quality Control, Microbiology, Production, Engineering, Quality assurance, IT, and HR for cGMP as per their respective checklist (Self Inspection Checklists).
-
- The auditors shall do the inspection as per the checklist and write their comments in the respective column along with the proposals for corrective actions wherever applicable and preventive actions as applicable.
-
- The scope of the audit need not be limited to the checklists.
-
-
The focus of the self-Inspection shall be to-
-
-
-
- Identify deviations from current authorized procedures,
-
-
-
- Check the procedures for their revision and update,
-
-
-
- Detect any shortcomings in the implementation of cGMP,
-
-
-
- Detect the data integrity issues and to recommend the necessary corrective action and also
-
-
-
- Compliance to marketing authorizations of the product.
-
-
- Head QA shall be the authorized person to give a final decision if any dispute raised by auditor/auditee related to GMP or other legal requirements.
-
-
Categorization of Observations :
-
-
- Categorize the observed non-conformities during self-Inspection as Critical, Major, and Minor. Definitions of these categories are described below:
-
- Critical:
-
- A non-conformity that has produced or may result in a significant risk of producing a finished product that is seriously harmful to the users.
-
- Such non-conformity affects the purity, strength, and/or safety of a finished product.
-
- Major:
-
- Any deviation from the validated or established procedure, process, system, or practices which can impact the product quality system.
-
- Minor:
-
- Any deviation related to documentation from controlled SOP or GMP norms, which may result in a risk described under Critical or Major as above.
-
-
Preparation of the Inspection Report and Compliance :
-
-
- After completion of the Internal Audit, QA/Audit Team shall prepare the department-wise inspection report as per Annexure –V within 5 working days.
-
- QA shall send the Internal Audit report as per Annexure –V to the Head of Department for compliance.
-
- Inspection log shall be updated as per Annexure-VII
-
- A unique self-Inspection report number shall be provided by QA as SI /XX/YY/ZZ where.
-
-
- SI denotes self-inspection followed by a ‘/ ’.
-
-
-
- XX denotes the last two digits of the calendar year followed by a ‘/ ’.
-
-
-
- YY denotes Inspection No. i.e. 01 for 1st Inspection and 02 for 2nd Inspection.
-
-
-
- ZZ denotes department code.
-
-
-
The Department Head shall submit the compliance report as per Annexure-VI to QA within 30 working days.
-
-
- The actions which are not closed within the time frame Target completion date shall be provided.
-
- For major and Critical observations CAPA shall be taken as per SOP for Corrective and Preventive Action.
-
- Deviation shall be handled through SOP for Incident/Deviation.
-
- Change control shall be handled through SOP for Change Control Management (CCP).
-
- Where the implementation of proposed corrective action requires major capital investment, the respective department head, Head QA shall get the approval from management.
-
- If corrective action and preventive action are planned in a phased manner then the inspection report shall be kept open till the action is completed.
-
- Audit Team/QA shall take the regular follow-up from the concerned department head for open corrective action and preventive action till completion of open actions.
-
- After completion of the inspection compliance report, the concerned department shall submit the signed copy to Audit Team/QA.
-
- The audit team/QA shall verify the implemented corrective actions.
-
- Finally, Head QA shall review the inspection Compliance reports for closer inspection.
-
- Reports of self-inspection are confidential and strictly meant only for internal circulation and shall not be shared with outside agencies.
-
- However, if inspection reports are required in the context of any investigation by any regulatory authority same shall be issued after the approval of Head QA as per Annexure VI.
5.0 References:
-
- Drug and Cosmetic Act: Revised Schedule M
-
- WHO-TRS 961
-
- Volume 4: EU Guidelines to Good Manufacturing Practice Medicinal Products for
Human and Veterinary Use, Chapter 9 Self-Inspection.
- Volume 4: EU Guidelines to Good Manufacturing Practice Medicinal Products for
6.0 Annexure – Self Inspection:
Annexure I: Flow Chart for Self Inspection.
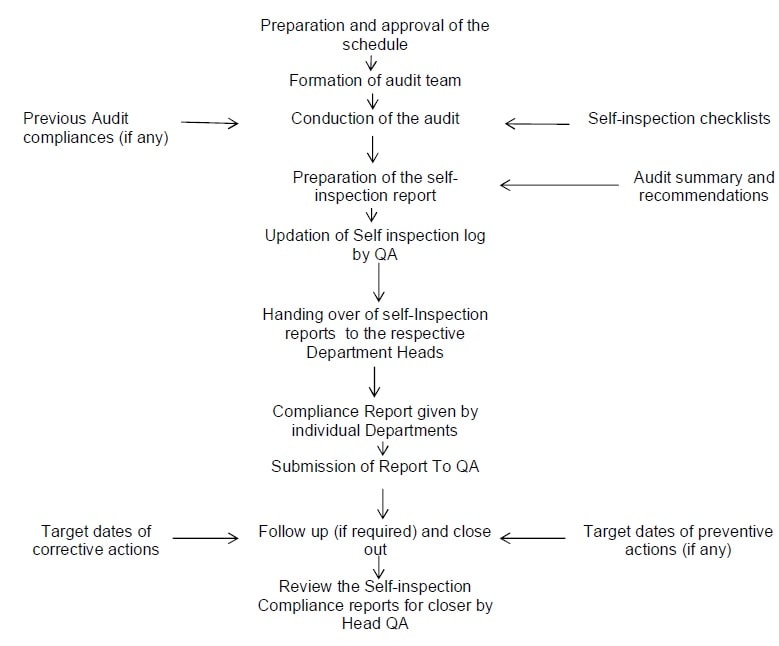
Annexure-II: Inspection Schedule.
For Year:………………………
| Department | Month | |||||||||||
| Jan. | Feb. | Mar. | Apr. | May | Jun. | Jul. | Aug. | Sep. | Oct. | Nov. | Dec. | |
Annexure III: Self-Inspection Team
For Year:
| Name | Department | Qualification | Experience |
Annexure-IV: Self-inspection summary report
Inspection Starting Date: ……………….. Closing Date:………………
Observations:
| Sr. No. | Observation | Critical | Major | Minor | References | Remarks |
| Department Name: | Date of Inspection | |||||
Summary:_____________________________________________________________
Recommendations:______________________________________________________
Annexure V: Self-Inspection Report
Inspection Report of……………………………..
Inspection Report No……………………………
Date of Inspection:……………………………
Name of Auditors:……………………………
Name of Auditee:……………………………
| Area | Observation | Category |
Annexure VI: Self Inspection Compliance Report
Inspection Compliance Report of……………………………..
Inspection Report Number………………………………….
| Sr. No. | Observation | Category | Corrective Action & Preventive Action | Status/ Target Date | Review Comments | Remarks |
Compiled By (Sign/Date)
Review Comments:
Annexure-VII: Self Inspection Logbook
| Date | Department | Inspection No. | Status | Target Completion Date | QA Sign/Date | Final Review | Sign & Date | Remarks
|
Annexure VIII: Request for issuance of Self Inspection report
| Inspection Report No. | |
| Period of Inspection | |
| Required for (Name of Authority) | |
| Reason | |
| Requested By (QA) | |
| Comment of Head QA
Date & Signature |
|
For the below Self Inspection Checklist (Department wise) please click the below links.
Department wise Self Inspection (Internal Audit) Checklist
-
- Stores & Ware House Inspection Checklist
-
- Production (OSD) Inspection Checklist
-
- Engineering Inspection Checklist
-
- Quality Control (Analytical) Inspection Checklist
-
- Quality Assurance Inspection Checklist
-
- Water System Inspection Checklist
-
- Microbiology Inspection Checklist
-
- HR / Administration Inspection Checklist
-
- Production (Oral Liquid) Inspection Checklist
-
- IT Inspection Checklist
Also Visit: Self Inspection (Internal Audit) Checklists
