Standard Operating Procedure (SOP), Guideline and Protocol for Process Validation and Verification for Drug Product (Tablet, Capsule etc.)
Process Validation (PV) Procedure
1.0 Objective :
-
- To lay down the procedure for Process Validation of pharmaceuticals products.
-
- Process validation provides documented evidence that a process is capable of reliably and repeatedly render a product of the required quality.
2.0 Scope :
-
- This SOP is applicable for process validation and process verification of Pharmaceuticals Products.
3.0 Responsibility :
| Chemist QA and above |
: |
- To prepare & review the process validation protocol & validation report.
- To draw samples as per validation protocol, and submit to Quality Control for analysis.
|
| Officer QC and above |
: |
- To analysis validation samples with the validated method & submit the results to Head Quality control.
|
| Head QC/Designee |
: |
- To review the process validation protocol & report.
- To update the laboratory for validation sample analysis & planning for analysis, interpretation the data of analysis or contract validation testing.
|
| Chemist Production and above |
: |
- To execute process as per routine production batches and as guided by validation protocol.
|
| Engineering |
: |
- To provide the supporting utility systems.
|
| Production Head/Designee |
: |
- To review the process validation protocol & report.
- To Plan & supervise manufacturing/validation activity.
|
| Head QA/Designee |
: |
- Approval of process validation protocol and report.
|
4.0 Procedure – Process Validation & Verification :
-
-
General Procedure for Process Validation
-
- Process validation shall be carried out for the drug products, which are being manufactured for the first time.
-
- Homogeneity within a batch and consistency between batches are goals of process validation activities.
-
- Process Validation shall consist of the manufacture of the product on the equipment specified for the purpose.
-
- Process validation shall include in-process control procedures to assure final product quality.
-
- Each step of a manufacturing process is controlled to assure that the finished product meets all quality attributes including specifications.
-
- The number of batches manufactured and the number of samples taken should be based on quality risk management principles, allow the normal range of variation and trends to be established and provide sufficient data for evaluation.
-
- An initial validation exercise with minimum three batches may need to be supplemented with further data obtained from subsequent batches as part of an on-going process verification exercise.
-
-
Prerequisites for Process Validation:
-
- Validation shall be performed for new manufacturing processes, equipment and when major changes have been made or implemented to premises, systems, equipment, materials and /or processes.
-
- Ensure that all relevant SOP’s are in place and training is completed on equipment operations, manufacturing instructions and sampling strategy are in place.
-
- Also that the operators and supervisory persons who will be running the validation batches have the understanding of the process.
-
- The process validation requires that method validation, calibrated instruments and qualified production support systems shall be established with proper documentation prior to execution of process validation studies.
-
- Furthermore, the process shall be fully developed, optimized and documented.
-
- Process validation shall focus on process performance at the set-point of each operating parameter range.
-
- When any new manufacturing formula or method of preparation is adopted, validation shall be done to demonstrate its suitability for routine processing.
-
- Validation can be prospective, concurrent or revalidation depending on when validation is performed but the type of validation shall be recorded in the respective validation protocol.
-
- In case of prospective or concurrent validation studies, the protocol shall specify a sufficient number of replicate process runs to demonstrate reproducibility and provide an accurate measure of variability among successive runs.
-
- The test conditions for the validation runs shall encompass upper and lower processing limits and circumstances, including those within standard operating procedures, which pose the greatest chance of process or product failure compared to ideal conditions.
-
-
In case of prospective and concurrent validation,
-
- The process validation protocol shall be written in a standard format that would enable the user to demonstrate that a process step, process condition, test requirement, or other relevant parameter or item that must be controlled within predetermined criteria to ensure that the product meet it specifications.
-
- The process validation protocol shall be prepared by the Quality Assurance department.
-
- The protocol shall be submitted for initial document approval prior to beginning of the execution in case of contract manufactured product.
-
Process validation involves a series of activities taking place over the lifecycle of the product and process as mentioned below:
-
Stage 1: Process Design Development :
-
- The commercial Manufacturing process is defined during this stage based on knowledge gained through development and scale-up activities that can consistently deliver a product that meets its quality attributes.
-
- A successful validation program depends upon information and knowledge from product and process development.
-
-
- Understand the sources of variation.
-
-
- Detect the presence and degree of variation.
-
-
- Understand the impact of variation on the process and ultimately on product attributes.
-
-
- Control the variation in a manner commensurate with the risk it represents to the process and product.
-
-
Stage II: Process Qualification/ Validation :
-
- During this stage, the process design is evaluated to determine if the process is capable of reproducible commercial manufacturing.
-
- This stage has two elements:
-
-
- Proper design of manufacturing facility
-
-
- Equipment must be of appropriate design, adequate size and suitably located
-
-
- Automated, mechanical and electronic equipment must be calibrated, inspected or checked.
-
-
- Utility systems and equipment are built and installed in compliance with the design specification and qualified (e.g. built as designed with proper materials, capacity, and functions, and properly connected and calibrated).
-
-
- Utility systems and equipment operate in accordance with the process requirements in all anticipated operating ranges.
-
- Process Performance Qualification:
-
-
- Greater scrutiny of process performance
-
-
Stage III: Continued Process Verification:
-
- An alternative approach to process validation in which manufacturing process performance is continuously monitored and evaluated.
-
-
Establishing a Monitoring Program:
-
- A Program of Continued Process Verification (CPV) provides a means to ensure that processes remain in a state of control following the successful process qualification stage.
-
- The information and data collected in stages 1 and 2, set the stage for an effective control strategy in routine manufacturing and a meaningful CPV program.
-
- The understanding of functional relationships between process inputs and corresponding outputs established in earlier stages in fundamental to the success of the CPV program.
-
- Continued monitoring of process variables enables adjustments to inputs covered in the scope of a CPV plan.
-
- It compensates for process variability, to ensuring that outputs remain consistent.
-
- Since all sources of potential variability may not be anticipated and defined in stage 1 and 2, unanticipated events or trends identified from continued process monitoring may indicate process control issues and / or highlight opportunities for process improvement.
-
- Science and risk-based tools help to achieve high levels of process understanding during the development phase, and subsequent knowledge management across the product life stage and facilities implementing continuous monitoring.
-
-
Documenting the Continuous Process Verification Program:
-
- A product-specific Continuous Process Verification (CPV) plan should include the following elements:
-
-
- Roles and responsibilities of various functional groups.
-
-
- Sampling and testing strategy.
-
-
- Data analysis methods (e.g., statistical process control methods).
-
-
- Acceptance criteria (where appropriate).
-
-
- Strategy for handling Out of Trend and out of specification
-
-
Continuous Process Verification data review:
-
- Continuous process verification is required during product life cycle either
-
-
- Introduction of new commercial product e. Stage IIIA
-
-
- Continued process verification for exiting product e. Stage IIIB
-
- The goal of this stage is continual assurance that the process remain in state of control (i.e. Validation stage) during the commercial manufacturing.
-
- A system(s) for detecting unplanned departure s from the process as designed is essential to accomplish this goal.
-
-
Evaluation of Post-PPQ batches of new commercial products i.e. Stage IIIA
-
- Batches manufactured during PPQ study shall be evaluated to get assurance of the process/product robustness and to identify the drifts, Refer the CPV program for new product, if there is not significant variability observed during PPQ study, New product shall be directly considered as routine batch and shifted in Stage IIIB and monitoring shall be done.
-
- If there was variability in any of the process parameter or Quality attributes as concluded in the process validation report then this stage III A is applicable to products
-
- If new products are developed according to QbD principles, the CQA, CPP, CMA and control strategy identified during development (Stage I, process design) are based on process understanding and quality risk management provided by the R&D for CPV monitoring.
-
- Strategy of all site shift products introduced at site first time shall be decided based on the conclusion made in the Stage-II i.e. in the process validation report of respective product.
-
- Protocol shall be designed accordingly.
-
- The number of batches requirement will be evaluated based on the outcome PPQ study that should identified in respective report based on the respective parameter variability and accordingly the sampling plan can be decided based on the statistical analysis.
-
- Based on the assessment/trend impact on the validated state shall be evaluated and accordingly improvement plan shall be finalized if required.
-
- Post monitoring, if process considered in state of control the routine batch release shall be proceeds with defined sampling plan and acceptance criteria.
-
-
Preparation of continuous verification protocol
-
- All new products become part of the CPV program stage-IIIA after their stage-II validation.
-
- The CPV protocol should be prepared as per product name, details and the protocol shall define the concept, the criteria and the scope of trending and reporting for identified parameters as concluded in the Stage-II.
-
- The protocol should be revised whenever one or more changes in process are made to establish new CPV limits if required.
-
- Selection of CQAs, CPPs and CMAs for monitoring:
-
- For new development product the specific CQAs, CPPs and CMAs are given by R&D Department based on risk assessment in product development report (PDR) and experience from Process validation.
-
- For all site shift product the critical process parameters and critical quality attributes included in the enhanced sampling and testing in Stage 2 should be considered initially for continued monitoring in Stage 3 based on the variability.
-
- With the appropriate risk-based analysis and documented justification (scientifically and statistically justified), certain Stage 2 parameters may be eliminated or the level of sampling testing could be reduced in the Stage III plan.
-
-
Number of batches for defining CPV limit :
-
- Data from a minimum of 30 batches after PPQ will be required, but number of batches can be reduce or increase based on the variability in the process parameter or results and with scientific rationale.
-
-
Evaluation and establishment of CPV limit
-
- When evaluating the performance of a process, it is often useful to set limits to provide an indication about when the variability of a parameter or attribute may be changing, and therefore, needs further attention.
-
- QA personnel shall enter the values of CPPs from executed BMR and QC personnel shall enter the values of CMAs and CQAs in worksheet form in analytical reports as required.
-
- The data should be statistically trended and reviewed by trained personnel (with adequate training in statistical process control techniques).
-
- While collecting the data of a minimum of 30 batches or as defined, if any change is made in manufacturing process through change management and such a change has an impact on critical attributes, then the process of data collection has to be restarted after the change is made effective and with proper justification, in order to establish CPV limits.
-
- The new set of data must cover a minimum of 30 batches.
-
- Where special causes for variations are identified, these values should be removed from calculations for the establishment of CPV limits.
-
- The real-time control (±3 sigma) shall be considered as limit before releasing of any batch.
-
- This control evaluation shall be started after production of 30 batches in order to collect sufficient data.
-
- Any outlying data shall be investigated.
-
- While it is possible that past data may fall as an outlier, this must be investigated and documented.
-
-
Report for freezing the CPV limit
-
- This should define the CPV limit of each attribute being monitored as identified.
-
- This should evaluate the process capability index of each attribute as identified.
-
- This report might suggest ways to improve and/or optimize the process by altering some aspect of the process or product, such as the operating conditions (ranges and set-points), process controls, component, or in-process material characteristics.
-
- When the root cause(s) has been determined for results which are out of CPV limits, then, for purposes of improvement of process, QA personnel together with subject matter expert/s shall take necessary corrective and/or preventive action(s) for such improvement.
-
- The report should assess the action plan for improvement of process, i.e. to check if the change(s) in process design require:
-
-
- Re-development of process.
-
-
- Re-process validation (verify control strategy).
-
-
- Reestablish process and sampling plan.
-
- Existing/legacy products developed traditionally may not have critical attributes or parameters defined in their submissions shall also be considered for process verification as per above procedure.
-
- If drug product/substance, not having defined CQAs, CPPs, and CMAs with PDR and PPQ reports, is adjudged to have a level of risk, the risk assessment shall be performed by a cross-functional team in order to identify CQAs, CPPs, and CMAs for monitoring in CPV.
-
- The assessment will be based on TT, engineering batch report, PPQ report, OOS, OOT, CC, audit outcome and deviations, product performance on stability, outcome of management review, based on APQR, and current process understanding.
-
-
Continued Process Verification for existing routine product i.e. Stage IIIB:
-
- The routine batch monitoring and release shall be done as follows (But Not Limited to)
-
-
Product Quality Review (PQR):
-
- PQR is an organized and comprehensive evaluation of all production, analytical and quality assurance data associated with drug products, done annually to identify needs for any corrective / preventive actions that would lead to product improvements and also ensure that the process remains in control.
-
- Maintaining the quality of incoming materials and components by means of a comprehensive testing program for same data of key incoming materials / components used for a product shall also be evaluated / trended to check for need for any corrective / preventive actions either as a part of the PQR process, or through a separate program.
-
- Timely assessment of Deviations, Changes, Out-of-Specification Results, Product Quality complaints by carrying out Investigations, Root cause Analysis and initiating CAPAs for same as well as trending of them periodically.
- Ongoing programs to collect and analyze product and process data that related to product quality with the help of trends, etc. i.e. In process and Finished product testing controls and OOT monitoring.
-
-
Maintenance of facility, Utilities and Equipment:
-
- Maintenance of a facility, Utilities and Equipment is an important aspect of ensuring that a process remains in a “State Of Control”.
-
- Once established qualification status shall be maintained through exhaustive preventive maintenance program, periodic Performance verification of equipment / utilities, routine monitoring program of facilities, Requalification program for utility.
-
- For all ongoing products continuous process verification shall be consider as PQR
-
- Detail APQR Procedure to be followed as per respective SOP .
-
Continuous process verification tools
-
- Continued Process Verification can be done using many tools and methodologies as mentioned PQR SOP
-
- Some of them are listed below:
-
-
- Graphical charts; for example, Control charts. Line charts can also be used as tools to determine whether a manufacturing process is in a state of statistical control.
-
- Statistical tools as explained below:
-
-
- Calculation of control limits
-
- The UCL and LCL shall be calculated as below:
-
-
- UCL (Upper Control Limit) = Average + 3σ
-
-
- LCL (Lower Control Limit) = Average – 3σ
-
-
- Where Average stands for mean and σ stands for standard deviation.
-
-
- For CPP and CQA with a single-side specification, the UCL and LCL shall be considered as below:
-
-
- Products having Upper Specification Limit, only UCL shall be considered
-
-
- Products having Lower Specification Limit, only LCL shall be considered
-
- If the calculated UCL and LCL are different from the specification limits, then specification limits shall be consider as UCL and LCL.
-
- Statistical process control indices which should be used are Cp (Process Capability), Cpk (Process Capability Index), Pp (Process Performance) and Ppk (Process Performance Index).
-
- Cp is a capability tracking mechanism, which compares the width of a product with variation with the process. This metric uses estimated standard deviation.
-
Formula for two-sided specification:
Cp = (Allowable Range) / 6 X SD i.e. (UQL-LQL) / 6 X SD
-
- Cpk uses estimated standard deviation to determine how well a system can meet the specification limits.
-
- It also takes the target value into account.
-
- Cpk shall be calculated for both side i.e. on UQL and LQL and minimum of both shall be considered as Cpk.
-
Formula for one-sided specification:
CpK (min), Lower = Estimated Mean – LQL / 3 X SD
CpK (max), Upper = UQL-Estimated Mean / 3 X SD
Where,
-
- Cpk: evaluate the centering of the process (minimum of CpK (min) and CpK (max))
-
- SD: Denotes standard deviation.
-
- UQL: Denotes Upper Quality level
-
- LQL: Denotes Lower Quality level
-
-
Acceptance criteria for Cp and Cpk:
-
- Cpk ‹ 1.0, then process is not capable.
-
- Cpk =1.0-1.33, Process is barely capable and in state of control with alert.
-
- If Cpk ≥1.33 Process is capable and in state of control.
-
- Same criteria are applicable for Cp.
-
- Pp shows process performance. It indicates well a system performs when it comes to upper and lower specification limits.
-
- However, it does not focus on the average and instead concentrates on the spread, formula for Pp is similar as Cp.
-
- Ppk uses actual standard deviation to determine process variation.
-
- The capability rate for Ppk is calculated using the formula below as per Cpk.
-
Types of Process Validation:
-
-
Prospective process validation:
-
- This shall be performed before an entirely new product is introduced by the company or when there is a change in the manufacturing process which may affect the product’s characteristics, such as uniformity and Identity etc.
-
- During product development the production process may be broken down into individual steps.
-
- Each step can be evaluated on the basis of experience or theoretical considerations to determine the critical factors/parameters that may affect the quality of the finished product.
-
- A series of experiments may be devised to determine the criticality of these factors.
-
- Representatives from production, QC/QA, and engineering development will normally be involved in this process.
-
- These experiments may incorporate a challenge element to determine the robustness of the process.
-
- Each experiment should be planned in an authorized and documented protocol.
-
- The criticality of these factors should be determined through a “worst-case” challenge where possible.
-
- Master Batch Documentation can be prepared/finalize only after the critical parameters of the process have been identified and machine settings, component specifications and environmental conditions have been determined.
-
- The batches/runs under validation should be documented comprehensively.
-
-
The following items should be included in the validation report:
-
-
- A description of the process – Batch/Packaging Document, including details of critical steps.
-
-
- A detailed summary of the results obtained from in-process and final testing, including data from failed tests.
-
-
- When raw data are not included reference should be made to the sources used and where it can be found.
-
-
- Any work done in addition to that specified in the protocol or any deviations from the protocol should be formally noted along with an explanation.
-
-
- A review and comparison of the results with those expected.
-
-
- Formal acceptance/rejection of the work by the team/persons designated as being responsible for the validation, after completion of any corrective action or repeated work.
-
-
Concurrent Process Validation:
-
- If concurrent validation approach shall be adopted, there should be sufficient data to support a conclusion that any given batch of product is uniform and meets the defined acceptance criteria.
-
- The results and conclusion should be formally documented and available to the Qualified Person prior to certification of the batch.
-
-
- It is important in concurrent process validation, however, that the premises and equipment to be used have been previously qualified and that the decision to carry out concurrent validation is made by authorized personnel.
-
-
- Documentation requirements are the same as specified for Prospective Validation and the testing to be carried out in-process and on the finished product will be as specified in approved protocols.
-
-
- The completed protocols and reports for the batch to be released should be reviewed and approved before product is released for sale or supply.
-
-
- This shall be carried out during routine production of products intended for sale.
-
-
Prospective & Concurrent Validation:
-
-
- The number of batches (minimum of 3) should be based on the variability of the process, the complexity of the process product and experience.
-
-
- Validation studies shall be carried out on minimum 3 consecutive batches of the product manufactured by the same manufacturing process, under identical conditions and set or equivalent of equipment controlling the critical process parameters on the basis of Process validation Protocol and tested for compliance to the pre-determined specification which also includes in-process sampling and testing,
-
-
- Process validation protocol specific the manufacturing conditions, control, testing and expected outcomes.
-
-
- A process validation protocol should be prepared which defines the critical process parameters (CPP), critical quality attributes (CQA) and the associated acceptance criteria which should be based on development data or documented process knowledge Process validation protocol shall include, but are not limited to the following
-
-
-
- A short description of the process and a reference to the respective Master Batch Record;
-
-
-
- Functions and responsibilities;
-
-
-
- Summary of the CQAs to be investigated;
-
-
-
- CPPs Summary and their associated limits;
-
-
-
- Summary of other (non-critical) attributes and parameters which will be investigated or monitored during the validation activity, and the reasons for their inclusion;
-
-
-
- List of the equipment/facilities to be used (including measuring/monitoring/recording equipment) together with the calibration/qualification status;
-
-
-
- List of analytical methods and method validation, as appropriate.
-
-
-
- Proposed in-process controls with acceptance criteria and the reason(s) why each in-process control is selected;
-
-
-
- Additional testing to be carried out with acceptance criteria;
-
-
-
- Sampling plan and the rationale behind it;
-
-
-
- Methods for recording and evaluating results;
-
-
-
- Process for release and certification of batches (if applicable).
-
Process evaluation and selection:
-
- Select and mention the processing steps needed for the initial scale-up in protocol are as follow but not limited to.
| Sr. No |
Oral liquid |
Tablet |
Capsule |
| 1. |
Physicochemical parameters
- Appearance
- pH
- Weight per ml
|
Dry Mixing :
- Mixing time
- Uniformity of Content (If applicable)
- Speed of impeller
|
Blending :
- Flow properties
- Moisture content/LOD
- Uniformity of Blend (If applicable)
|
| 2. |
Mixing:
- Determine and evaluate specification
- Mixed ingredients Appearance
- In-process testing
- Assay of the active ingredient
|
Granulation:
- Binder temperature
- Speed of impeller
- Speed of chopper
- Ampere load
- Time for addition of binder
- Moisture content/LOD
|
Filling of Capsules
- Powder flow from hopper
- Height of Powder in Hopper
- Speed of Filling Machine
- All physical characteristics i.e. Average Fill Weight, Uniformity of Fill weight, Disintegration / Dissolution, locking of capsules etc
|
| 3. |
Filling and sealing
- Uniformity of volume
- Sealing of the bottle
- Presence of foreign particles.
- Homogeneity, wherever required
|
Drying
- Optimal LOD of the dried granulation
- Air Flow, Inlet Temperature, and Outlet Temperature & Load to be dried.
- Racking time and intervals
- Drying time
|
Packing:
- Forming Temperature
- Sealing Temperature
- Machine Speed
- Sealing i.e. Leak Test
- Coding Details
|
| 4. |
—– |
Blending
- Mixing Time
- RPM of blender (If applicable)
- Flow properties
- LOD
- Uniformity of blend (If applicable)
|
—– |
| 5. |
—– |
Tablet (core) compression
- In-process checks at variable machine speed & Compaction force (Hardness)
- Height of powder in hopper
|
—– |
| 6. |
—– |
Coated tablets
- Wight gain, Inlet temperature, Bed Temperature, Pan Speed, Peristaltic pump, Spray rate & cycle, Solution stirring, Atomizing Air pressure.
- Pan negative pressure, Distance between Gun & bed.
|
—– |
| 7. |
—– |
Packing:
- Forming temperature
- Sealing Temperature
- Machine Speed
- Sealing i.e. Leak Test
- Batch Coding Details (embossing and printing )
|
—– |
-
- The drug having dose proportional formula shall be validated once up to common stage and all subsequent stage shall be validated separately.
-
- Individual quantifiable acceptance criteria will be pre-defined and specified for all process critical parameters in the individual protocols.
-
Revalidation Criteria:
-
- Once a process has been validated, it is considered to be in a state of control.
-
- As long as all conditions and control parameters remain unchanged, the process continues in its validated state.
-
- Consideration for the need revalidation, whenever there is change in, Revalidation shall be considered under following considerations and it should be initiate through change control procedure.
-
-
Revalidation shall be carried out for following cases:
-
-
- Change in Manufacturing (Procedure and/or process)
-
-
- Raw materials change (physical properties such as density, viscosity, particle size etc.)
-
-
- Change in or modification of critical equipment.
-
-
- Change in facility and installations, which influence the process.
-
-
- Manufacturing area or site change.
-
-
- Transfer of processes to another site or unit.
-
-
- As per the recommendations In Change Control form, Investigation reports or complaint evaluation and on appearance of negative quality trends, if required.
-
-
- On the basis of annual product review, self inspection, trend analysis or non conformance.
-
- The revalidation study shall be carried out as per the protocol prepared for individual activity.
-
- The revalidation protocol and report shall prepared as per procedure described for prospective and concurrent validation
-
- This includes rationale for critical process parameters and critical in process parameters including the worst case scenario consideration or challenge study to be performed for the selection of critical process parameters as per SOP for Quality Risk Assessment.
-
- Rationale for Critical Process Parameters: This shall indicate the rationale for the selection of critical process parameters.
-
- Critical in process controls: This shall define the rationale for the selection of critical in process parameters used in the process validation study.
-
- Worst case scenario or challenge study: This shall include the challenges during the process validation considering the probable failures or worst case parameters without affecting the quality attributes.
-
Sampling Plan and Acceptance Criteria:
-
- The validation team shall schedule the validation program & inform the respective departments about the program.
-
- The validation team shall consist of staff members from Production, Quality Control, Engineering and Quality Assurance.
-
- Validation activity shall be performed by validation team.
-
- The responsibility of each department shall be mentioned in the respective validation protocol.
-
- Process parameters that could affect the critical quality attributes of the product shall be identified.
-
- Key parameters shall be frozen in Process validation protocol and associated batch records before undertaking process validation exercise.
-
- Range for each critical process parameters expected to be used during routine manufacturing and process control shall be determined.
-
- All the equipment/instrument to be used shall be checked for the qualification & calibration status. Qualification & calibration status shall be mentioned in report.
-
- The validation program shall only be started after the confirmation of the equipment and instrument status.
-
- During the process validation extensive sampling and testing shall be performed on the product at various stages and shall be documented comprehensively.
-
- The sampling plan, including sampling points, number of samples, and the frequency of sampling for each unit operation and attribute is defined in respective protocol.
-
- The number of samples taken during validation shall be adequate to provide sufficient statistical confidence of quality both within a batch and between batches.
-
- The extent of sampling, tests and acceptance must take into consideration the level of risk.
-
-
Sampling Plan and Acceptance Criteria shall be as per below table:
| Stage |
Sampling Plan |
For Tablet
|
| Dry Mix ( If applicable) |
- Sample from at least five different locations in triplicate evenly distributed throughout the mixer (e.g. RMG)
- Sample Size 1X – 3X (Where X: Avg. Wt. of single dose unit)
- Send one set for analysis to QC and two set shall be reserved with QA (for investigation of OOS/OOT if required.)
- Sample shall be destroyed after completion of report.
|
| Drying |
- One sample shall be analyzed after each drying or as defined in protocol.
|
- At least 1 samples from different locations after final drying process as per sample plan.
|
| Pre-blend or Final Blend / Mix |
- Sample from at least ten different locations in triplicate evenly distributed throughout the blending (e.g. Bin Blender).
- Sample Size 1X – 3X (Where X: Avg. Wt. of single dose unit)
- Send one set for analysis to QC and two set shall be reserved with QA (for investigation of OOS/OOT if required.)
- Sample shall be destroyed after completion of report.
|
- Composite sample for QC analysis as per protocol
|
| Compression |
- Speed challenge, Hopper Level Study and Hardness (Compaction) challenge as per protocol.
- Sample quantity shall be sufficient for complete testing of in-process parameter.
|
- Composite sample for QC analysis as per protocol
|
| Coating |
- Composite sample for QC analysis as per protocol
|
| Primary packaging |
- Speed challenge and temperature challenge shall be performed as per protocol.
|
| Finished Product |
- Sampling shall be done throughout packing process as per protocol.
|
For Liquid
|
| Before Filtration or Sieving |
- Samples from Upper Layer, Middle Layer, Lower layer & Mixed sample. (E.g. Manufacturing Tank). Sample Size: 100- 200ml
- As per process validation protocol (if applicable)
|
| After Filtration or Sieving |
- Composite sample from (e.g. Storage Tank)
- Sample Size: 100- 200ml as per process validation protocol (if applicable)
|
| Filling, Sealing & Labeling |
- Sampling as per process validation protocol
|
| Finished product |
- Sampling as per process validation protocol
|
For Capsule
|
| Pre-blend or Final Blend / Mix |
- Sample from at least ten different locations in duplicate evenly distributed throughout the blending (e.g. Bin Blender).
- Sample Size 1X – 3X (Where X: Avg. Wt. of single dosage unit)
- Send one set for analysis to QC and one set shall be reserved with QA (for investigation of OOS/OOT if required.)
- Sample shall be destroyed after completion of report.
|
|
|
| Filling & Polishing |
- Speed challenge and Hopper Fill Study shall be carried out as per process validation protocol
|
| Primary packaging |
- Speed challenge and temperature challenge shall be performed as per protocol.
|
| Finished Product |
- Sampling shall be done throughout packing process as per protocol.
|
|
|
|
|
-
-
Procedure for Sampling and Analysis:
- Sampling shall be done by trained QA person as per the sampling plan described in validation protocol.
-
- QA shall send the sample with intimation as per Annexure-V to QC department for analysis as per protocol.
-
- QC Personnel shall receive the sample with intimation.
-
- After complete analysis QC department shall provide the complete report with raw data to QA.
-
- Validation data for all the critical parameters shall be collected & evaluated against predetermined set of limits.
-
- A report of the validation shall be prepared by QA in computerized format but not limited to defined contents in annexure.
-
- Contents shall be changed as per requirement.
-
- Summary report detailing the critical validated product/machine parameter that is to be followed in routine production in future shall be prepared & conclusion drawn from it shall be mentioned in the report.
-
-
Recommendation of process validation report shall be shared with user department.
-
- Any exceptional conditions encountered during Process Validation shall be investigated and the appropriate course of action (justification, correction, or re-qualification studies) determined.
-
- Any deviations or exceptions to approved protocols shall be noted, investigated and resolved and if required CAPA shall be initiated and shall be executed and closed.
-
- On completion of validation batches study, the respective documents shall be revised to incorporate the changes in operational parameters if recommended In the Process Validation report through change control procedure.
-
- If a change is proposed in any of the procedures, processes, or equipment, which may impact the quality then that, shall be done by following change control procedures.
-
- All changes must be formally requested, documented and accepted by the Validation Team and Quality Assurance department.
-
- The proposed changes shall be scientifically assessed and based on assessment need of re-validation or revision of document shall be determined.
-
- After the confirmation that the process design is capable of reproducible commercial manufacture, continuous process verification of the process is carried out as per recommendation of process validation report (If applicable).
-
Release of Process Validation Batch:
-
- Validation batches shall be released after reviewing the Batch Manufacturing & Batch Packing Record.
-
- Analysis results shall be reviewed and should be complying with the specification.
-
- Interim report shall be prepared if three consecutive batches are not manufactured.
-
- However, such batches shall remain under monitoring until accelerated stability studies are completed.
-
- Stability samples from the process validation batches shall be drawn and kept for stability study as described in the stability protocol.
-
- Process Validation protocol and validation reports along With attached documents shall be filed and stored In Quality Assurance Department.
-
- Process Validation Planner shall be prepared as per Annexure No. VI and updated annually.
-
- Ongoing Process validation/ verification detail shall be updated in Annexure No. VII
5.0 Reference (S)
-
- Working Document QAS/03.055/REV.2
-
- Validation Guidelines for Pharmaceutical Dosage Forms (GUI-029)
-
- Volume 4, EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and
- Veterinary Use, Annex 15: Qualification and Validation
-
- Validation Master Plan Installation and Operational Qualification Non-Sterile Process Validation Cleaning Validation (PI 006-3).
-
- WHO Technical Report Series, No. 937, Annex-4, Appendix-7
6.0 Annexure (S)
Annexure I : Flow diagram for process validation
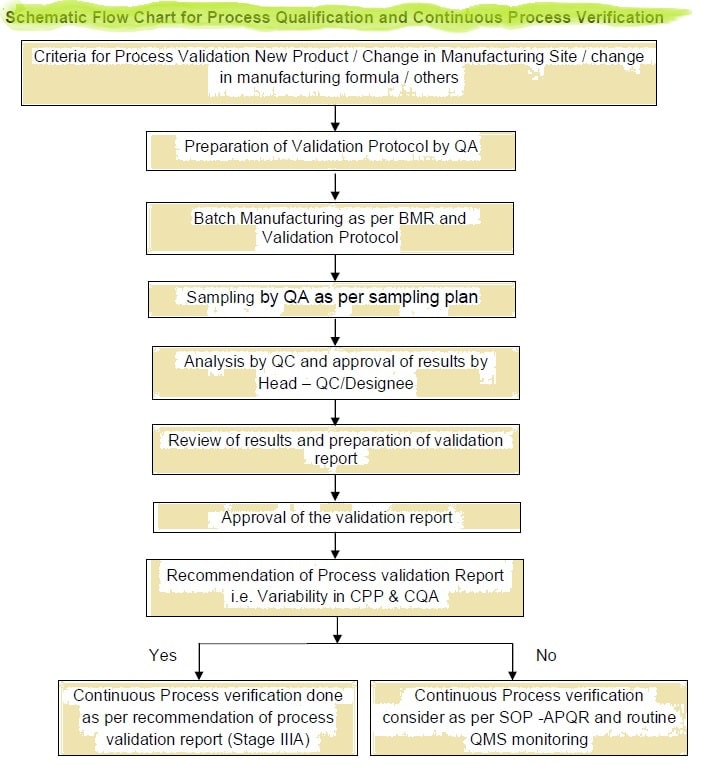
Annexure II : Process Validation Protocol Number Issuance log
|
Sr. No.
|
Date |
Product Name |
Batch size |
Protocol No. and effective date |
Issued By
|
|
|
|
|
|
|
|
|
|
|
|
|
| Checked by |
Report No. & Effective date |
Issued by |
Checked by |
Remark |
|
|
|
|
|
|
|
|
|
|
Annexure III : Template for Process validation Protocol
| Sr. No. |
Subject |
Page No. |
| 1. |
Table of Contents |
|
| 2. |
Protocol Approval |
|
| 3. |
Objective |
|
| 4. |
Scope |
|
| 5. |
Responsibilities |
|
| 6. |
Validation Criteria |
|
| 7. |
References |
|
| 8. |
Product Profile |
|
| 9. |
Description of Equipments to be used |
|
| 10. |
Weightment sheet of Materials |
|
| 11. |
Process Flow Chart |
|
| 12. |
Sampling Plan |
|
| 13. |
Methodology of Sampling |
|
| 14. |
Abbreviation |
|
| 15. |
Schematic diagram of equipment |
|
| 16. |
Deviation/OOS (if any) |
|
| 17. |
Enclosures |
|
| 18. |
Revision History |
|
Annexure IV : Template for Process validation Reports
| Sr. No. |
Subject |
Page No. |
| 1. |
Table of Contents |
|
| 2. |
Approval |
|
| 3. |
Objective |
|
| 4. |
Scope |
|
| 5. |
Responsibility of Validation Team |
|
| 6. |
Batch Details |
|
| 7. |
Training of Validation Team |
|
| 8. |
Description of Equipments used |
|
| 9. |
Summary of critical process parameters |
|
| 10. |
Packing Details |
|
| 11. |
Results of Finished Product |
|
| 12. |
Yield |
|
| 13. |
Deviation Report (If any) |
|
| 14. |
Summary Report |
|
| 15. |
Conclusion |
|
| 16. |
Recommendation if any |
|
Annexure V : Sample intimation Slip
| Product |
|
Batch No. |
|
Batch Size |
|
| Mfg. Date |
|
Exp. Date |
|
Date |
|
| Stage |
|
| Sample for analysis for :- |
| Test |
√ (done) & x (not done) |
No. of Samples |
Sample collected by QA |
Sample received by QC |
| Description |
|
|
|
|
| Physical Parameters |
|
| Uniformity of Content |
|
| Dissolution |
|
| Disintegration |
|
| Water / LOD |
|
| pH |
|
| Wt. per ml |
|
| Assay |
|
| Microbial test |
|
| Additional Test |
|
|
|
|
|
|
|
|
|
|
|
| Report Received By (QA) |
Annexure VI : Process Validation Planner
Process Validation Planner ….. Document No.: ……..
Section: Dosage Form: Effective Date:
| Sr. No. |
Product Name |
Composition |
Reason for validation |
Market |
Batch No. |
Batch size |
Validation Status |
Remark |
| 1. |
|
|
|
|
|
|
|
|
| 2. |
|
|
|
|
|
|
|
|
| 3. |
|
|
|
|
|
|
|
|
| Particulars |
Prepared By |
Reviewed By |
Approved By |
| Sign. |
|
|
|
| Date |
|
|
|
Annexure VII : Ongoing Process Validation/ Verification Detail
| Sr. No. |
Product Name |
Composition |
Reason for validation |
Market |
Batch No. |
| 1. |
|
|
|
|
|
| 2. |
|
|
|
|
|
| 3. |
|
|
|
|
|
| Sr. No. |
Batch size |
Validation Status |
Updated by (sign./Date) |
Checked by (sign./Date) |
Remark |
| 1. |
|
|
|
|
|
| 2. |
|
|
|
|
|
| 3. |
|
|
|
|
|
| Particulars |
Verified By(Sign./ date) |
Approved By |
| Sign. |
|
|
| Date |
|
|
*********************************************END*********************************************

