Standard Operating Procedure (SOP) and Protocol for Transport Validation through temperature mapping during transit of drug products.
Transport Validation Procedure
1.0 Objective
-
- To lay down the procedure for conducting transport validation and to describe acceptance criteria for the study.
-
- The purpose of this SOP is to provide detailed steps to conduct transport validation and to evaluate the product integrity and effect of the environmental condition as temperature and relative humidity during transport on the quality of Finished Pharmaceutical Product dispatched.
2.0 Scope
-
- This SOP is applicable for Finished Pharmaceutical Products manufactured at the drug manufacturing location.
3.0 Responsibility
-
-
Quality Assurance:
-
-
- To prepare and review the protocol for transport validation.
-
- To issue the protocol.
-
- Ensure data loggers are calibrated.
-
- To compile and review the data of transport validation.
-
- Engineering:
-
- To facilitate the calibration of the data logger.
-
- Warehouse:
-
- Placement of data loggers in the consignment.
-
- Perform a pre-shipment visual inspection of consignment.
-
- To activate the data loggers.
-
- Ensure the pre-shipment visual inspection of containers.
-
- To download data from the data loggers after receipt
-
- Head – Quality Assurance
-
- To approve the transport validation protocol and report.
-
- Customer / Consignee.
-
- To check the physical condition of the consignment.
-
- Record the details in the data logger tracking sheet.
-
- To send the data logger or temperature and relative humidity data to the plant.
4.0 Procedure – Transport Validation :
-
-
Transport Validation Approach:
-
-
- To review the transport route for suitability by the means of assessment of environmental conditions (temperature and relative humidity) on the product including product integrity during transport.
-
- To ensure that products can be safely transported within the transport temperature profile defined for each product and that compliance can be demonstrated to the regulatory authorities and other interested customers.
-
-
Definitions
-
-
- Temperature excursion: A temperature excursion is a variance outside of the labeled storage conditions.
-
-
Revalidation Criteria
- The transport revalidation shall be done in case of:
-
-
-
- Change in route of transportation.
-
-
-
- For new transporter.
-
-
-
- Change in the port of destination.
-
-
-
- Any recommendation by customer.
-
-
-
- Whenever temperature and/or humidity monitoring shows unexplained variability that is greater than normal.
-
-
- The extent of transport revalidation shall depend on the significance and nature of changes, which shall be evaluated by QA
-
Procedure For Transport Validation:
-
- The validation team for the transportation study shall consist of personnel from QA, Engineering warehouse, and marketing (wherever required).
-
- QA shall ensure that persons carrying out the transport validation must be trained on respective operations/procedures (SOPs).
-
- QA shall ensure that the data logger is calibrated as per SOP.
-
- Warehouse person shall inform QA about the details such as the introduction of new transporter, name of the transporter, vehicle number, and vehicle size, product details such as the name of the product, batch number, and the number of packed shippers at least for one day in advance before dispatch.
-
- QA shall verify the products and transport details and evaluate that transport validation is required or not.
-
- If temperature validation study is applicable on the transport vehicle, QA shall communicate to all the concerned persons from the site and depot/marketing department regarding the same.
-
- Data logger shall be used as per customer requirements.
-
- QA shall provide the transport validation study number and record in Annexure-VII.
-
-
Transport validation study number shall be provided as below:
-
TV/YYNNN
‘TV’ stands for ‘Transport Validation’.
‘YY’ stands for the last two digits of the current year.
‘NNN’ is a sequential number starting from 001, 002, and so on.
For Example- TV/21001 indicates that the first Transport validation performed in the year 2021.
-
- QA shall issue the controlled copy of the transport validation protocol as per Annexure-I and report as per Annexure-II to the warehouse.
-
- Warehouse person shall arrange the shippers/pallets/drums of product which is under transport validation.
-
- The warehouse person shall perform a visual inspection of all the containers to be shipped for cleanness and any defects and record the observations in Annexure-III.
-
- If the transport container is dirty and/or damaged, then wait until unless it is cleaned and/or rectified.
-
- QA shall ensure the pre-shipment visual inspection of containers along with the warehouse person.
-
- Quality Assurance shall ensure that packed products are complying with the approved specifications.
-
- QA shall calculate the dimension of the vehicle (i.e. length and width) which is provided by the warehouse and divide the area into a grid of approximately ’10 feet X 10 feet (i.e. 100 square feet).
-
-
If the area is smaller than 100 square feet, consider it as one grid.
-
-
- Arrange a minimum of three data loggers for each grid in case of without Palletization and four data loggers in case of with Palletization.
-
- QA shall check the battery level of each data logger, preferably the battery level shall be 100% but shall not be less than 90% level (if applicable).
-
- In case, data loggers are having a battery level near 90% then QA shall provide a spare battery to the transporter and make him understand to change the battery if required.
-
- The warehouse person shall switch on the data logger and set the program in data loggers to record the temperature and relative humidity.
-
- The warehouse person shall set a minimum of 30 minutes frequency interval to take the reading in the data logger.
-
- Data loggers shall be placed while loading of shippers and same shall be recorded in Annexure-VI.
-
-
In case of without pallet, warehouse person shall place the data logger in Identified shipper / drum (upper corner of shipper drum) of the consignment in presence of QA.
-
-
- When shipper without pallet shall be dispatched and Palletization to be done at outside of manufacturing site then a communication shall be send to depot / marketing department as ‘only one shipper labeled as ‘CONTAINS DATA LOGGER’ shall be placed on one pallet.
-
- In case of with pallet, warehouse person shall place the data logger on upper corner on half of the total pallets and on lower corner on half of the pallets of the consignments in presence of QA.
-
- Warehouse person shall label the outer surface on each side of shipper / pallet / drum which contains the data logger as ‘CONTAINS DATA LOGGER; as per site SOP for labeling.
-
- QA shall verify the functioning and placement of data logger in the shipper / pallet / drum.
-
- QA shall ensure that temperature and relative humidity of container keeps at lower side of required range and must not cross the specified range during loading shipper of pallets in to containers.
-
- Real time photograph of loading pattern shall be attached with validation report by QA.
-
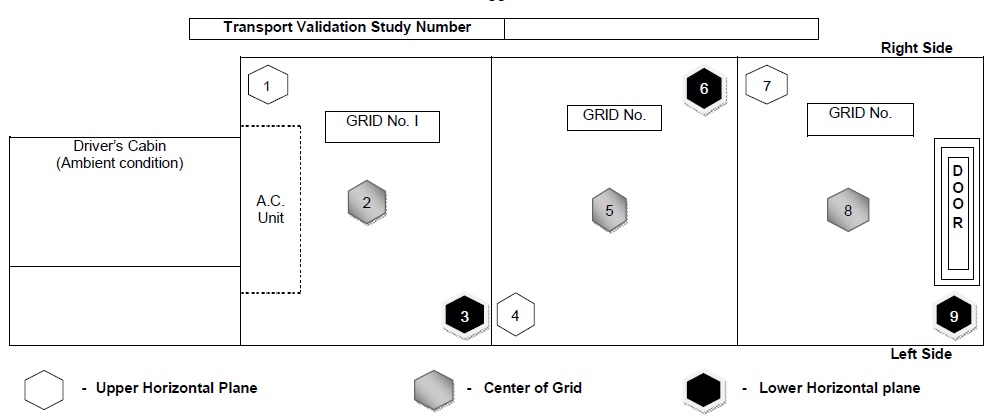
- Diagrammatic representation of the position of data logger in the vehicle and data logger number to be recorded in Annexure-IV for without pallet and in Annexure-V for with pallet.
-
-
The rationale for selection of diagonal location to place data loggers in containers when shippers are without pallet is as below :
-
| S. No. | Grid No. | Data Logger Location | The rationale of selection of location |
| 1 | I | Right side – Upper level | Behind the A.C. unit, Difficult to achieve the desired temperature |
| 2 | I | Centre – Middle level | The location is surrounded by shippers in all directions so the desired temperature is difficult to achieve. |
| 3 | I | Left side – Lower Level | To demonstrate temperature distribution in the lower horizontal plane |
| 4 | II | Left side – Upper Level | To demonstrate temperature distribution in the upper horizontal plane |
| 5 | II | Centre – Middle Level | The location is surrounded by shippers in all directions so the desired temperature is difficult to achieve. |
| 6 | II | Right side – Lower Level | To demonstrate temperature distribution in the lower horizontal plane |
| 7 |
III |
Right side – Upper level | To demonstrate temperature distribution in the upper horizontal plane |
| 8 |
III |
Centre – Middle level | The location is surrounded by shippers in all directions so the desired temperature is difficult to achieve. |
| 9 |
III |
Left side – Lower level | To demonstrate temperature distribution in the lower horizontal plane and at location farthest away from the A.C. unit |
-
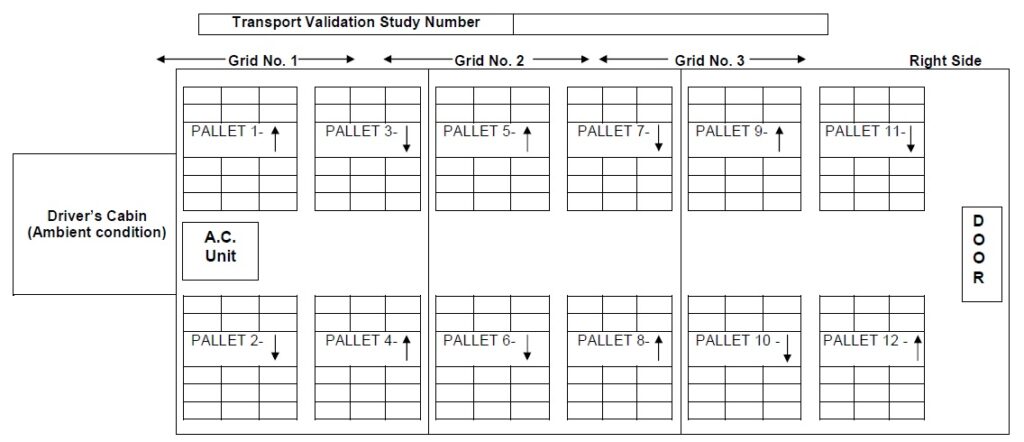
- In the case of shippers with pallets, one data logger on each pallet to be located diagonally so as to demonstrate that temperature is uniformly distributed, throughout the vehicle.
-
- QA shall intimate the Export department/marketing department/consignee/customer to return the data logger immediately on receipt of the consignment at their end to plant by courier.
-
- Uncontrolled copy of the approved protocol, with Annexure-IV or Annexure-V (Location of data logger in the container) and Annexure-VI (Datalogger tracking sheet), shall be sent to the consignee either with the shipment or through the mail (scanned copy) or both by warehouse person.
-
- The warehouse person shall seal the container and the seal number shall mention in Annexure-VI.
-
- After completion of packing, the shipment shall be sent to the customer’s destination.
-
- Upon receipt of shipment at customer destination (depot), a customer representative shall Checks / confirms the physical conditions of shipment and fill the details in the data logger tracking sheet.
-
-
Verification of physical condition and product integrity includes (but not limited to)
-
-
-
- Wet products
-
-
-
- Damaged products
-
-
-
- Tempered products
-
-
- (If required) withdraws samples from the shipper/pallet/drum in which data logger is placed (quantity required for two-time analysis).
-
- If the data logger is button operated then the customer representative shall STOP the data logger and shall send the data logger to the plant along with Annexure-VI and COA of the analyzed sample (if samples withdrawn by the customer representative).
-
- Upon receipt of the data logger to plant along with Annexure-VI and COA of analyzed sample (if samples withdrawn by customer representative), warehouse person shall stop the data logger if the data logger is software operated and shall collect the data from data loggers.
-
- QA shall attach these documents (data from data logger, data logger tracking sheet, and COA of the analyzed sample) with the protocol.
-
- QA shall review the results of analyzed samples and evaluate the compliance.
-
-
The analytical results and data from the data logger shall be compiled in a report and attached to the protocol.
-
-
- After review and evaluation of data, summary of transport validation shall be approved and shared to customer / marketing person.
-
- In case, the data logger is provided by customer then temperature and relative humidity data shall be provided by customer to plant.
-
- Any deviation such as temperature excursion or product damage has occurred during transportation, product fails during analysis and departure from protocol instruction shall be recorded and investigated as per SOP for deviation handling.
-
- Product stability data must demonstrate the acceptable temperature excursion time during transport.
-
- Risk assessment of delivery routes shall be used to determine where temperature controls are required.
-
- A risk assessment shall be performed to consider the impact of variables in the transportation process other than those conditions which are continuously controlled or monitored, e.g. delays during transportation, failure of monitoring devices and any other relevant factors. This risk assessment shall be attached to validation report.
-
- In case of data, loggers lost or data loggers get STOPPED in between transport, then the transport validation study shall be considered as invalidate and required to repeat.
-
Acceptance Criteria:
-
- There must not be any damage to the product during transport.
-
- The analytical results of the product must comply with the release specification (if applicable).
-
- The temperature and relative humidity data must be within the specified range.
5.0 Annexure (S):
Annexure I: Protocol for Transport Validation
-
Objective:
- To ensure that the temperature controlled transport vehicle is capable of maintaining vehicle is capable of maintaining the temperature and relative humidity within the specified limit throughout the transit duration from plant to customer depot.
-
Scope :
-
- This protocol shall be applicable for the finished products which are manufactured and supplied by
-
- This protocol shall be applicable for India and Export market.
-
- Justification For Selection of product for Transport Validation
Few justifications are as follow, but not limited to:
-
- Change in route of transportation.
-
- Change in port of destination.
-
- Environment conditions changed of transport.
-
- Change in environment conditions of transport.
-
- Any recommendation by customer.
-
- Temperature and/ or humidity monitoring shows unexplained variability that is greater than normal.
-
Site of Validation:
- Pharma manufacturing site to customer depot
-
Team Details:
- Representatives from manufacturing site from below departments
- Warehouse
- Engineering
- Quality Assurance
- Representatives from manufacturing site from below departments
-
Details of transport vehicle and product:
- Details of Transport Vehicle:
- Name of transporter, GMP agreement number, vehicle number, vehicle size.
- Details of Transport Vehicle:
-
- Details of Product:
- Name of product, batch number, storage condition, number of shippers, loading pattern (with pallet/ without pallet)
- Details of Product:
-
SOPs to be followed:
- SOP for use of data loggers
- Loading of shippers
- SOP for deviation handling
-
Controls:
- Calibrated data loggers shall be used
-
Training:
- Personnel carrying out the validation shall be trained on respective (SOPs)
-
Procedure – Transport Validation:
- Warehouse person shall inform to QA about the details such as new transporter, name of transporter, vehicle number, and vehicle size, product details such as name of the product, batch number and number of packed shippers at least one day in advance before dispatch.
-
- QA shall verify the products and transport details and evaluate that transport validation is required or not.
-
- If temperature mapping Study is applicable on the transport vehicle, QA Shall communicate to all the concern persons from site and depot regarding the same.
-
-
For Export, data logger can be used as per contract giver or customer consignment.
-
-
- QA shall issue the certified copy of transport validation protocol as per Annexure- I and report as per annexure-II.
-
- Warehouse person shall arrange the shippers/ pallets/ drums of product which is under transport validation.
-
- Warehouse person shall perform a Visual inspection of all the containers to be shipped for cleanness and any defects and record the observations in Annexure- II
-
- If transport container is dirty and/ or damaged, then wait until unless it is cleaned and / or rectified.
-
- QA shall ensure the pre-shipment visual Inspection of containers along with warehouse person.
-
- Ensure that packed products are complying with the approved specifications.
- QA shall measure the dimension of the vehicle (i.e. length and width) and divide the areas into grid of approximately 10 feet X 10 feet (i.e. 100 square feet). If areas is smaller than 100 square feet, consider it as one grid.
-
-
Arrange minimum three data loggers for each grid.
-
-
- Place at least one data logger in driver’s cabin for recording the ambient condition.
-
- QA shall switch on the data logger and set the program in data loggers to record the temperature and relative humidity.
-
- Data loggers shall be placed while loading of shippers and same shall be recorded in Annexure-V.
-
- In case of without pallet, warehouse person shall place the data logger in identified shipper / drum (upper corner of shipper drum) of the consignment in presence of QA.
-
- In case of with pallet, warehouse person shall place the data logger on upper corner on half of the total pallets and on lower corner on half of the pallets of the consignment in presence of QA.
-
- Warehouse person shall label the outer surface on each side of shipper / pallet / drum which contains the data logger as “CONTAINS DATA LOGGER” as per site SOP for labeling.
-
-
QA shall verify the functioning and placement of data logger in the shipper / pallet / drum.
-
-
- Real time photograph of loading pattern shall be attached with validation report by warehouse person.
-
- Diagrammatic representation of the position of data logger in the vehicle and data logger number to be recorded in Annexure-III for without pallet and in Annexure-IV for with pallet.
-
- QA shall intimate Export department / consignee / customer to return the data logger immediately on receipt of the consignment at their end to manufacturing site or Export Department by courier.
-
- A copy of the approved protocol, with Annexure-V shall be sent to the consignee either with the shipment or through mail (scanned copy) or both by warehouse person.
-
- After Completion of packing, the shipment shall be sent to the customer destination from manufacturing site.
-
- Upon receipt of shipment at customer destination (depot), a customer representative shall Checks / confirms the physical conditions of shipment and fill the details in data logger tracking sheet.
-
-
Verification of physical condition and product integrity includes (but not limited to)
- Wet products
- Damaged products
- Tempered products
-
-
- (If required) withdraws samples from the shipper / pallet / drum in which data logger is placed (quantity required for two time analysis).
-
- Customer representative shall STOP the data logger and shall send the data logger to manufacturing site along with Annexure-V and COA of analyzed sample (if samples withdrawn by customer representative).
-
- Upon receipt of the data logger to manufacturing site along with Annexure-V and COA of analyzed sample (if samples withdrawn by customer representative), QA shall collect the data from data loggers.
-
-
QA shall attach these documents (data from data logger, data logger tracking sheet and COA of analyzed sample) with protocol.
- QA shall review the results of analyzed sample and evaluate for compliance.
-
-
- The analytical results and data from data logger shall be complied in a report and attached to protocol.
-
- After review and evaluation of data, summary of transport validation shall be approved and shared to customer.
-
- In case, the data logger is provided by customer then temperature and relative humidity data shall be provided by customer to manufacturing site.
-
- Any deviation such as temperature excursion or product damage has occurred during transportation, and departure from protocol instruction shall be recorded and investigated as per SOP – Deviation.
-
- Product stability data must demonstrate the acceptable temperature excursion time during transport.
-
- Risk assessment of delivery routes shall be used to determine where temperature controls are required.
-
Acceptance criteria:
- There must not be any damage of product during transport.
-
- The analytical results of product must comply with the release specification.
-
- The temperature and relative humidity data must be within the specified range.
- Deviation:
-
- Details of deviation (including justification of acceptance, if any) shall be recorded.
- Summary of findings of validation:
-
- To be recorded in the validation report.
- Conclusion and Recommendations:
-
- To be recorded in the validation report.
- Approval:
-
- After completion of validation study, report shall be approved by quality head.
- Attachments:
- All the filled forms and supporting data shall be attached with report.
- Abbreviations:
| CFR | Code of Federal Regulation |
| QA | Quality Assurance |
| QC | Quality Control |
| COA | Certificate of Analysis |
| Risk Assessment | A systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across the product lifecycle. |
Annexure II: Report for Transport Validation
-
Objective:
- To ensure that the temperature controlled transport vehicle is capable of maintaining the temperature and relative humidity within the specified limit throughout the transit duration from manufacturing Site to customer depot.
-
Scope :
- This report shall be applicable for the finished products which are manufactured and supplied by
-
- This report shall be applicable for India and Export market.
- Justification For Selection of product for Transport Validation:
____________________________________________________
_________________________________________________________
- Site of Validation:
-
- Manufacturing Site_________________________________
-
- Customer depot_________________________________
- Team Details:
-
- Representatives from manufacturing site from below departments:
| Department | Designation | Name | Sign | Date |
| Warehouse | ||||
| Engineering | ||||
| Quality Assurance | ||||
- Details of transport Vehicle and Product:
- Details of Transport Vehicle:
| Sr. No. | Items | Details |
| 1. | Name of transporter | |
| 2. | GMP Agreement number | |
| 3. | Vehicle Number | |
| 4. | Vehicle Size (Length in ft.) |
- SOPs to be followed:
-
- Use of data loggers (SOP)__________________________________________
-
- Loading of shippers (SOP)__________________________________________
-
- Deviation handling___________________________________________
- Controls:
- Calibration details of data loggers
| S. No. | Data logger ID | Calibration done on | Calibration due on |
| 1. | |||
| 2. | |||
| 3. | |||
| 4. | |||
| 5. |
-
Training:
| S. No. | Name of Person | Department | Training done on date | Checked by QA |
- Procedure:
-
- Carry out the study as per procedure given in Protocol.
- Observation:
-
- Refer Annexure-IV- Data logger tracking sheet
-
- Data retrieved from data logger
-
- Refer COA of samples (if available)
- Acceptance Criteria:
-
- There must not be any damage of product during transport
-
- The analytical results of product must comply with the release specification
-
- The temperature and relative humidity data must be within the specified range.
-
Deviation:
| Sr. No. | Deviation No. | Deviation Details | Status | Justification of acceptance |
- Summary of Observations of Validation:
____________________________________________________
_________________________________________________________
- Conclusion and Recommendations:
____________________________________________________
_________________________________________________________
-
Approval:
| Functional Area | Name | Signature | Date |
-
Attachments:
| Sr. No. | Details of attachments |
| 1. | Pre-shipment Visual Inspection of Containers |
| 2. | Locations of data loggers placed in vehicle |
| 3. | Risk assessment |
| 4. | |
| 5. |
- Abbreviations:
| CFR | Code of Federal regulation |
| QA | Quality Assurance |
| QC | Quality Control |
| COA | Certificate of Analysis |
Annexure III: Pre-shipment visual inspection of containers
| Transport Validation Study number | |||||
| Sr. No. | Check point | Vehicles
No.: |
Vehicles
No.: |
Vehicles
No.: |
|
| 01 | Transport Name | ||||
| 02 | Vehicle Type | AC/Non Ac/Cold
van |
AC/Non Ac/Cold
van |
AC/Non Ac/Cold
van |
|
| 03 | Vehicle Temperature | Before loading
———-0C After loading ———-0C |
Before loading
———-0C After loading ———-0C |
Before loading
———-0C After loading ———-0C |
|
| 04 | Check Cleanliness of vehicle from inside (Ceiling/roof ,walls and floor) | OK/NOT OK | NOT OK/OK | OK/NOT OK | |
| 05 | Check Cleanliness of vehicle from outside | OK/NOT OK | NOT OK/OK | OK/NOT OK | |
| 06 | Check Cleanliness of floor (below the tarpaulin in case of non AC vehicle) | OK/NOT OK | NOT OK/OK | OK/NOT OK | |
| 07 | Vehicle is free from odor | YES / NO | NO/YES | YES / NO | |
| 08 | Check absence of any abnormal fitting, foreign material | NOT OK/OK | OK/NOT OK | OK/NOT OK | |
Annexure-IV: Locations of data loggers placed in vehicle without pallet
Annexure V: Locations of data loggers placed in vehicle with pallet
Annexure VI: Data logger tracking sheet
| Transport Validation Study Number | ||||||
| Description | Observation | |||||
| Shall be filled by Manufacturing Site | ||||||
| Storage condition on the pack | ||||||
| Product in which Data logger is placed | ||||||
| Batch No. in which Data Logger is placed | ||||||
| Other products if any sent along with the consignment | ||||||
| B. No’s of Other products if any. | ||||||
| Total Number of container shipped | ||||||
| Total Number of container shipper with data logger | ||||||
| Type of Packing | Normal / Cold box | |||||
| Expiry Date / Use Before Date | ||||||
| Time interval set for taking readings in data logger | ||||||
| Under temperature set in data logger (Lower Limit) | ||||||
| Over temperature set in data logger (Upper Limit) | ||||||
| Seal Number (for transport container) | ||||||
| Shipper / pallet / Drum No. on which loggers are affixed and data logger No. | ||||||
| S. No. | Shipper / Pallet/ Drum No. | Data Logger No. | S. No. | Shipper / Pallet/ Drum No. | Data Logger No. | |
| 1 | 7 | |||||
| 2 | 8 | |||||
| 3 | 9 | |||||
| 4 | 10 | |||||
| 5 | 11 | |||||
| 6 | 12 | |||||
Description |
Observation |
||
| Shall be filled by Manufacturing Site | |||
| Quantity Shipped / Number of shippers / Pallets / Drum | |||
| Name of transporter and vehicle number | Transporter:
Vehicle No.: |
||
| Initial temperature of consignment
(where consignment is stored) |
Date : Time:
Temperature: |
||
| Initial temperature of refrigerated vehicle
(if applicable) |
Date : Time:
Temperature: |
||
| Set temperature of the vehicle (in case of refrigerated vehicle) | |||
| Date and Time on which data logger is kept in the consignment | S. No. | Data Logger No. | Date & Time |
| 1 | |||
| 2 | |||
| 3 | |||
| 4 | |||
| 5 | |||
| 6 | |||
| 7 | |||
| 8 | |||
| 9 | |||
| 10 | |||
| 11 | |||
| 12 | |||
Description |
Observation |
| Shall be filled by Manufacturing Site | |
| Time and temperature of door opening of refrigerated vehicle for consignment loading | Date : Time:
Temperature: |
| Time and temperature of door closing of refrigerated vehicle for consignment loading | Date : Time:
Temperature: |
| Date and time of dispatch | Date : Time:
|
| Expected Transit Time in days | Sea / Air / Road |
| Filled by (QA)- Name / Signature / Date | |
| Checked by (QA)- Name / Signature / Date | |
| Description | Observation | |||
| Shall be filled by consignee / customer (Depot) | ||||
| Date and Time of Receipt of the consignment | Date : Time: | |||
| Data Logger is in the specified Shipper / Pallet / Drum | Yes/No | |||
| Date and Time on which data logger is taken out from Shipper / Pallet / Drum | S. No. | Data Logger No. | Date & Time | |
| 1 | ||||
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 | ||||
| 6 | ||||
| 7 | ||||
| 8 | ||||
| 9 | ||||
| 10 | ||||
| 11 | ||||
| 12 | ||||
| Physical Condition of product / product Integrity: ________________________________________________
(Please verify and comment on product conduction including satisfactory or wet product / damaged product / tempered condition or any other) |
||||
| Checked by- Name / Signature / Date |
|
|||
| Return of Data Logger | ||||
| (This Column details shall be filled by site prior to send consignment )
Please return the Data Logger to the respective Manufacturing Site Address : Name of the concerned person : Contact Number |
|
| Description | Observation |
| Shall be filled by Manufacturing Site | |
| Receipt of Data Logger by Quality Assurance | Date : Time:
|
| Quality Assurance Evaluation and closure statement
(Retrieved data to be enclosed) |
|
| Checked by (QA)
Name / Signature / Date |
|
Annexure VII: Transport Validation Study Log
| Date | Transport Validation Study number | Reason for Study | Product Name | Batch Number | Country | Logged by QA (Sign & Date) | Results of Study | Reviewed By QA (Sign & Date) |
6.0 Reference (S):
-
- WHO Technical Report Series 961, 211 –Annex9
-
- European Commission Guideline on good Distribution Practice of medicinal products for human use (2013/C 343/01)
