Guideline for Technology Transfer. Tech Transfer is a systematic procedure that contains the transfer of any process together with its documentation and professional expertise between development and manufacture or between manufacture sites to produce similar quality products.
It is a systematic procedure that is followed in order to pass the documented knowledge and experience gained during development and or commercialization to an appropriate responsible and authorized location.
Technology Transfer Procedure
1.0 Objective :
- To provide a procedure for all activities performed by R&D department during technology transfer and to ensure that required information, data and technology about the product, processing and analysis is transferred to the manufacturing unit and available at site so that quality and integrity are maintained.
2.0 Scope :
- This Guideline is applicable for the technology transfer of drug products for regulated market as follows:
- New product technology transfer
- Approved product transfer
3.0 Responsibility :
| Head R & D / PDL | : |
|
| Head Production / Designee (RU) | : |
|
| Head Production / Designee (SU) | : |
|
| Head Supply Chain Management | : |
|
| Head Purchase | : |
|
| Head QC | : |
|
| Head QA / Designee (RU) | : |
|
| Head QA / Designee (SU) | : |
|
4.0 Procedure – Guideline for Technology Transfer :
-
What is Technology Transfer:
- A systematic procedure that contains the transfer of any process together with its documentation and professional expertise between development and manufacture or between manufacture sites to produce similar quality products.
- It is a systematic procedure that is followed in order to pass the documented knowledge and experience gained during development and or commercialization to an appropriate responsible and authorized location.
- Technology transfer is considered successful if a receiving unit can routinely reproduce the transferred product process or method against a predefined set of specifications as agreed with a sending unit.
-
Sending Unit (SU):
- The manufacturing site / research center where a designated product process or method is currently developed or manufactured and is expected to be transferred to other location
-
Receiving unit (RU):
- The manufacturing site where a designated product process or method is expected to be transfer.
-
New product:
- New product means a finished dosage form e.g tablet, capsules or Oral Liquid etc that contains a drug generally, but not necessarily, in association with one or more other ingredients developed or manufactured to get regulatory approval in respective countries.
-
Approved product:
- Approved product means a listed drug approved by respective regulatory agency for manufacturing, marketing, sale and distribution directly or indirectly to retail class of trade with labeling packaging or repackaging, product code.
-
Requirements for technology transfer:
- Transfer of technology requires a planned approach using trained and knowledgeable personnel working within a robust quality system, with documentation of data covering all aspects of production and quality control.
- Plant QA shall identify the impact on system /procedure due to new product inclusion at plant and shall purpose to update site master file, product list, validation master plan, equipment cleaning matrix and related documents as per SOP for Introduction New Product.
- The SU shall assess the suitability and degree of preparedness of the RU before transfer, with regard to premises, equipment and support services (e.g purchasing and inventory control mechanism, Quality control (QC) procedures, documentation computer validation, site validation, equipment qualification, water for pharmaceutical use and waste management).
- The analytical method transfer shall be carried out.
- In case of any utility or facility is not available at site modifications are to be made shall be done in consultation with Head – Operation, Head PDL and competent person of engineering.
- In case during scale up batches, any change or modification in formulation, equipment and machinery is required, same shall be done as per SOP for Change control.
- During the execution of validation batches, if any modifications in process/material/equipment is required it should be documented as deviation and its investigation report shall be captured in validation report
- The validation batches shall be charged for stability as per stability protocol.
- Storage of exhibit/validation batches shall be under the control of respective ware house.
- The SU and RU shall jointly implement any training programme that may be required specific to the product, process or method to be transferred, e.g on analytical methods or equipment usage, and assess training outcomes.
-
Initiation of Technology Transfer:
- Project/product technology transfer shall be initiate a project/ technology transfer initiation form (Annexure-I).
- Product transfer initiation responsibility shall be as follows…
| Technology Transfer Type | Responsible Department |
| PDL to manufacturing location | Head PDL or designee |
| Product transfer from Location to Location | Head marketing or designee /DRA |
- Technical transfer initiator shall complete the product transfer activities in coordination with the coordination of SU and RU.
- A unique project identification number shall be issued by PDL group.
- Number shall be recorded in project number issuance register (Annexure-II).
- Each project (Product technology transfer) number shall be assigned a 10 digit unique tracking number as follows:
- PTT/YY/ZZZ
- Where,
- PTT stands for Product technology transfer
- YY stands for last digit of year 20 for 2020
- ZZZ stands for serial number starting from 001.
-
New Product Technology Transfer (From PDL to Manufacturing Location) :
- Product Development Lab (PDL) shall initiate new product introduction as per SOP for New Product Introduction.
- PDL shall initiate change control for the introduction of the new product to the respective site.
- PDL shall provide the new product details to QA (RU) for approval product permission / Form 29 from the local regulatory authority.
- QA (RU) shall apply and get the product permission / Form 29 from the local regulatory authority.
- Tooling procurements can be carried out based on the approved tool diagrams.
- Process demonstration through scale up batch manufacturing can be carried out at manufacturing location and destruction of the same shall be done by respective manufacturing head or designee.
- PDL shall prepare all necessary documents such as master formula record, bill of material for RM & PM and stability protocol, punch tooling diagram & specifications etc.
- QA shall review all the documents and authorize the same.
- API, excipients, in-process, finished product specifications and test procedure etc. shall be prepared by PDL.
- PDL shall send raw materials & packaging materials shortages to ware house (RU). Ware house (RU) shall convey the requirement of raw material & packaging material to purchase team for procurement
- Ware house (RU) shall receive all the / packaging materials to be used in the exhibit batch /test batch manufacture following the same systems/procedures as followed for any input material for regular batch.
- Before finalization of step’s analytical method validation shall be completed by QC and analytical method shall be transferred.
-
The detail responsibilities for document preparation have been given in Annexure-III.
- PDL shall prepare BMR/BPR after the receipt of MFR, RM, BOM of RM & PM, In-process specifications, scale up report and get it approved from process development, production, drug regulatory affairs and quality assurance department.
- The master copy of the BMR/BPR shall be submitted to QA for archiving controlling and issuance of the documents for manufacturing of the batch.
- The procedure for issuance of all related to exhibit batch/test batch manufacture shall be same as that of the regular production batches.
- Validation / QA (RU) shall prepare validation protocol/ monitoring protocol, Hold time study protocol, sampling plan, cleaning validation protocol (if required)/Equipment cleaning verification matrix in coordination with PDL & QC.
- Before start of technology transfer manufacturing QA shall ensure the availability of all required documents mentioned in check list (Annexure -IV).
-
If some documents are pending or needed corrections same shall be initiated.
- Proper justification /correction of documents shall be captured in the checklist.
- All cross-functional teams shall coordinate with each other for planning execution of exhibit batches as per the market requirement.
- All the raw/packaging materials required for the manufacturing of the batches shall be issued as per procedure adopted for regular production batches.
- A record of all the dispensed material shall be maintained.
- Production /PDL shall manufacture exhibit batches as per regulatory requirement in presence of PDL and QA.
- RU QA / validation shall monitor product technology transfer activity, shall be protocol bound, study and the results shall be complied as a report and ensure cGMP compliance during the execution of batches
- The details of the input materials, environmental conditions during manufacturing process followed for product manufacture and packaging yield / reconciliation of packaging material at various stages details of in-process sampling and monitoring reconciliation of packing materials and final release of the batch shall be documented in detail in the respective BMR/BPR.
-
PDL shall check the BMR/BPR when the batches are manufactured in PDL.
- Production (RU) shall fill & check the BMR/BPR when the batches are manufactured in facility and PDL shall monitor the manufacturing and packaging when executed in production facility.
- In process and finished product samples must be collected by QA (RU) for scale up, exhibit batch, validation batches as per the sampling plan to assure that the batch is of desired quality.
- The executed BMR/BPR shall be reviewed by PDL/production /QA, after review, any discrepancy found in the BMR/BPR same shall be recorded.
- After successful completion of exhibit batches, samples shall be kept for stability as per stability protocol.
- Where bio studies are required, plant QA shall withdraw the samples and send for study through PDL / DRA.
- After verification of all the necessary documents QA shall close the change control.
- After submission of samples for bio-equivalence /bio-availability, stability and other purpose, the batch shall be stored under recommended storage conditions.
- Statement detailing the issuance of samples for different purposes shall be maintained by warehouse .
- If the batch is to be destroyed a clearance for destruction shall be obtained from regulatory department/PDL.
-
The destruction shall be done as per the existing procedure of that production head.
- The batch manufacturing and packing record of the exhibit batch shall be under the custody of QA and records shall be retained.
- Intended BMR/BPR shall be prepared by PDL (if required) by considering the exhibit batch parameters.
- These documents should be reviewed and approved by QA (RU) and DRA. QA (RU) shall forward these BMR’s/BPR’s to DRA for respective submission.
- In case of any change in the manufacturing process or formula revision of document shall be done through change control procedure.
-
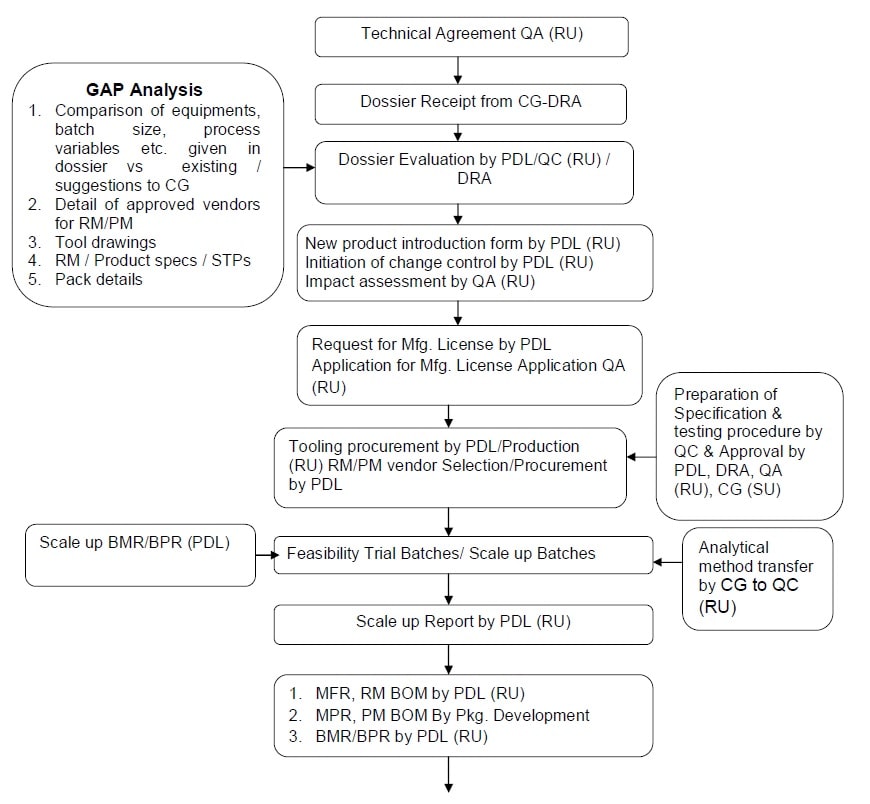
Approved product technology transfer: Product transfer from Contract Giver to Company
- There shall be a technical agreement between the parties, which specifies the responsibilities before, during and after technology transfer.
- QA (RU) shall made technical agreement with contract giver.
- QA (RU) & DRA shall provide the product dossier from contract giver PDL.
- Dossier evaluation / Gap analysis shall be done by PDL, QC (RU), packaging development (RU) and DRA.
- PDL shall initiate new product introduction.
- Product Development Lab (PDL) shall initiate change control for the introduction of the new product.
- PDL shall provide the new product details to QA (RU) for applying product permission / Form 29 from the local regulatory authority.
- QA (RU) shall apply and get the product permission / Form 29 from the local regulatory authority.
- Cross functional team shall coordinate with each other to plan validation batches form contract giver.
- PDL/Production shall done raw materials and packaging material vendor selection.
- PDL/Production shall send raw materials & packaging materials shortages to ware house (RU).
- Ware house (RU) shall convey the requirement of raw material & packaging material to purchase team for procurement.
- Ware house (RU) shall receive all the / packaging materials to be used in the exhibit batch /test batch manufacture following the same systems/procedures as followed for any input material for regular batch.
- Warehouse shall take the special care that the materials are stored under stipulated storage condition.
- Contract giver in coordination with QC (RU) shall do analytical method transfer to QC (RU).
- If required scale up batch / feasibility trial batches, PDL shall prepare scale up BMR, sampling plan and execute the scale up batch / feasibility trial batch.
-
All the activities of followed process shall be done by PDL.
- Sampling of scale up batch according sampling plan shall be done by QA (RU).
- In-process and finished product samples shall be analyzed by QC (RU).
- PDL shall prepare scale up report / feasibility trail batch report on the basis of data generated from scale up batch.
- Product Development Lab (PDL) shall share the scale up report / feasibility trail batch report with contract giver (SU).
- PDL shall prepare MFR and upload the BOM in electronic system.
- The BOM shall be approved by QA (RU).
- QC (RU) shall prepare rest of document like RM & PM, In-process specifications, test procedures and stability protocol as per dossier received.
- QA (RU) shall send all TT related documents to stake holder.
- PDL shall prepare BMR/BRP and get it approved from QA.
- Validation / QA (RU) shall prepare validation protocol/ monitoring protocol, Hold time study protocol, sampling plan, cleaning validation protocol (if required)/Equipment cleaning verification matrix in coordination with PDL & QC.
- Before start of technology transfer manufacturing QA shall ensure the availability of all required documents mentioned in Annexure-IV.
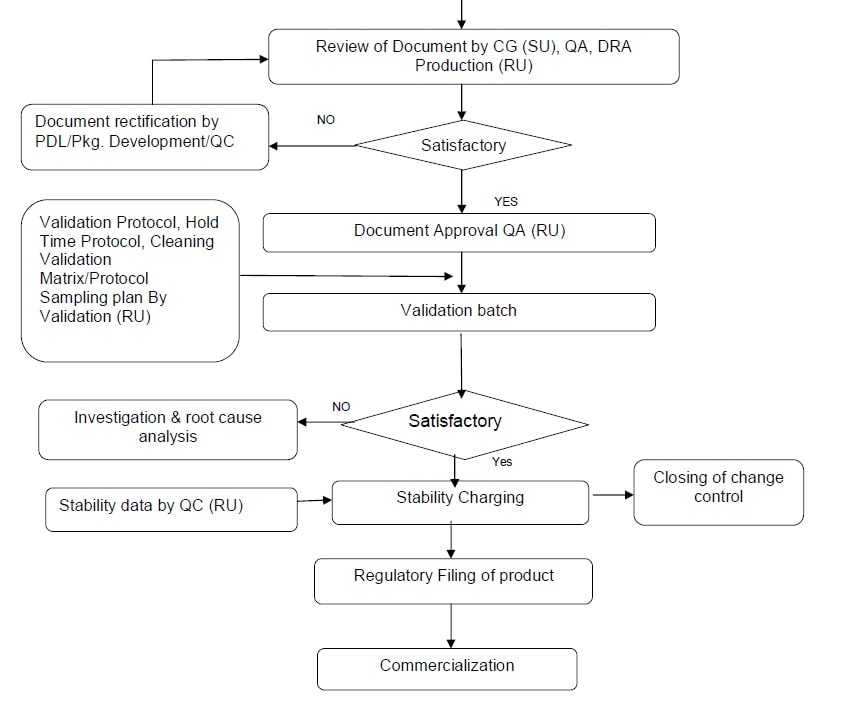
- If some documents are pending or needed corrections same shall be initiated by QA (RU) to respective department. Proper justification /correction of documents shall be captured in the checklist.
-
After verification of all the necessary documents QA (RU) shall approved the change control.
- Production shall manufacture exhibit batches / validation batches in presence of PDL and QA. as per regulatory requirement in presence of PDL and QA.
- The details of the input materials, environmental conditions during manufacturing process followed for product manufacture and packaging yield / reconciliation of packaging material at various stages details of in-process sampling and monitoring reconciliation of packing materials and final release of the batch shall be documented in detail in the respective BMR/BPR.
- RU QA / validation shall monitor product technology transfer activity, shall be protocol bound, study and the results shall be complied as a report and ensure cGMP compliance during the execution of batches
- In process and finished product samples shall be collected by QA (RU) as per the sampling plan to assure that the batch is of desired quality.
- The executed BMR/BPR shall be reviewed by PDL/production /QA, after review, any discrepancy found in the BMR/BPR same shall be recorded.
- After successful completion of exhibit batches, samples shall be kept for stability as per stability protocol.
- Post validation changes for process, formula, batch size and others will be handled by Production (RU) and QA (RU) in consultation with PDL and DRA through change control process. Document revision shall be carried out by Production (RU) / QA (RU).
- In case of any change in packing process or packs, revision of BPR shall be done through change control process by Production (RU) / QA (RU).
-
Technology Transfer Completion Report:
- PDL with QA (RU) shall prepare technology transfer compilation report based on data collected from process validation batches.
- Refer flow chart as per Annexure-V.
5.0 Reference (S) :
- WHO technical report series No. 961, 2011, Annexure 7.
6.0 Glossary – Guideline for Technology Transfer:
- PDL : Product Development Laboratory
- TT : Technology transfer
- BOM : Bill of materials
- WHO : World Health Organization
- RU : Receiving Unit
- SU : Sender Unit
- CG : Contract Giver
- BMR : Batch Manufacturing Record
- BPR : Batch Packaging Record
- MFR : Master Formula Record
- DRA : Drugs Regulatory Affairs
- API : Active Pharmaceutical Ingredient
7.0 Annexure (S)
Annexure – I : Project /Technology Transfer Initiation Form
| Product Name | ||
| Generic Name | ||
| Strength(s) | ||
| Technology Transfer (Mention existing and proposed site) | ………………………………………………. to …………………………………… | |
| Label Claim(s) | ||
| ANDA/MA/Registration No. | ||
| Market(s) | ||
| Expected Market Volume | ||
| Nature of Technology Transfer | New Product / Approved Product | |
| Reason of site change or addition | ||
| Project Approval | ||
| Department | Comments | Signature and date |
| Initiator | ||
| Initiating department head | ||
| Head QA | ||
Annexure – II : Project Number Issuance Register
|
Project No. |
Name of Product | Strengths | Technology Transfer | Market | Initiator |
Remarks |
|
|
From |
To |
||||||
Annexure – III : Responsibilities for New Product Introduction
| Sr. No. | Activity / Documents | Responsibility |
| 1. | Technical Agreement | QA (RU) |
| 2. | Receipt of technology transfer dossier from Contract Givers | DRA / PDL / QA (RU) |
| 3. | TT Initiation form | PDL |
| 4. | New Product Introduction | PDL / QA (RU) |
| 5. | Change control Initiation | PDL |
| 6. | License detail to the manufacturing location | Contract Giver / PDL |
| 7. | Application for manufacturing license to state Food and Drug Administration (FDA) | QA (RU) / PDL |
| 8. | Facility requirement review for equipment, building, Infrastructure etc. | PDL / Production / Engineering / QA |
| 9. | Indent for purchase of equipment, tooling, change parts etc. | PDL / Production |
| 10. | Installation, qualification of new equipment | Production / Engineering / QA / Validation |
| 11. |
Manufacturing of scale up or feasibility or trial batches |
PDL / Engineering / QA / Validation /Contract Giver |
| 12. | Preparation RM/PM Specification, product specifications, review and approval | QC (RU) / QA (RU) |
| 13. | Preparation and approval / Authorization of MFR , MPR & BOM | PDL / Pkg. Development /QA (RU |
| 14. | Maintenance of approved vendor list | QA (RU) |
| 15. | Approval of contract testing lab | QC / QA (RU) |
| 16. | Purchase of RM/PM | PDL / Ware house / Purchase |
| 17. | Vendor qualification documents with TSE/BSE declaration | QA (RU) |
| 18. | Sampling / Analysis / Release of RM & PM | QC(RU) / QA (RU) |
| 19. | Analytical technology transfer at location | Contract givers / QC (RU) |
| 20. | Receipt of RM / PM | Ware house (RU) |
| 21. | Sampling, analysis, release of finished products | QC /(RU) / QA (RU) |
| 22. | Preparation, checking & approval of BMR / BPR | PDL / DRA / Production / QA |
| 23. | Updation of cleaning validation matrix | Validation / QA / QC |
| 24. | Preparation and approval of stability protocol | QC (RU) / DRA / Contract Giver |
| 25. | Preparation and approval of process monitoring / validation protocol / report | Validation / PDL / Production / Engineering /QA |
| 26. | Execution of the validation batches | PDL / Production / QA |
| 27. | Validation batch process monitoring / validation | PDL / Production / Validation QA / QC |
| 28. | Execution of cleaning validation as required | Production / Validation / QA / QC |
| 29. | Review or executed BMR/BPR | Production / DRA / QA |
| 30. | Stability study monitoring | QC |
| 31. | Retention / destruction of samples of batches | Production / QA / Warehouse |
| 32. | Retention of documents | QA |
| 33. | Equipment equivalency | PDL / QA |
Annexure – IV : Technology Transfer Execution Checklist
| Project No. | |
| Name of Product | |
| Strength(s) | |
| Market |
| Sr. No. | Documents | Comments by QA | Remark (if any) |
| 1. | TT Initiation form | ||
| 2. | NPI Approval | ||
| 3. | Change control number & Approval | ||
| 4. | Manufacturing License | ||
| 5. | MFR | ||
| 6. | Bill of material of RM | ||
| 7. | PM BOM | ||
| 8. | Stability protocol & any other protocol | ||
| 9. | PM specification and STP | ||
| 10. | Lay outs of packing components | ||
| 11. | Punch tooling Diagram / Capsule filling change parts | ||
| 12. | Raw material specification and STP | ||
| 13. | Excipients Specification and STP | ||
| 14. | In-Process, Finished product Specification and STP | ||
| 15. | RM / PM Availability | ||
| 16. | Compression / Capsule filling change parts | ||
| 17. | API Validation Package | ||
| 18. | Finished Product Analytical Method Validation | ||
| 19. | VSR (TSE/BSE/RS declaration etc) | ||
| 20. | Document completion certificate | ||
| 21. | QC standards | ||
| 22. | Impurity profile | ||
| 23. | Reference standards | ||
| 24. | Impurity standards | ||
| 25. | Cleaning method | ||
| 26. | BMR / BPR | ||
| 27. | Sampling protocol | ||
| 28. | Validation protocol | ||
| 29. | Cleaning validation protocol | ||
| 30. | Equipment Qualification | ||
| 31. | Hold time study protocol | ||
| 32. | Pharmacopoeial Update | ||
| 33. | ECOM code Creation if any |
Annexure – V : Flow Chart